5.1.17. intersect¶
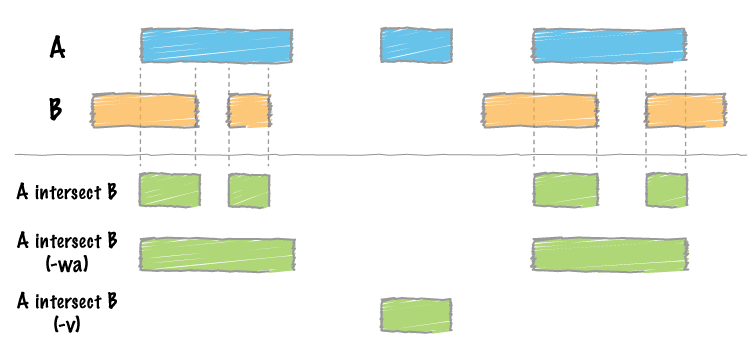
By far, the most common question asked of two sets of genomic features is whether or not any of the features in the two sets “overlap” with one another. This is known as feature intersection. bedtools intersect allows one to screen for overlaps between two sets of genomic features. Moreover, it allows one to have fine control as to how the intersections are reported. bedtools intersect works with both BED/GFF/VCF and BAM files as input.
Note
If you are trying to intersect very large files and are having trouble with excessive memory usage, please presort your data by chromosome and then by start position (e.g., sort -k1,1 -k2,2n in.bed > in.sorted.bed for BED files) and then use the -sorted option. This invokes a memory-efficient algorithm designed for large files.
5.1.17.1. Usage and option summary¶
Usage:
bedtools intersect [OPTIONS] -a <BED/BAM/GFF/VCF> -b <BED/BAM/GFF/VCF>
(or):
intersectBed [OPTIONS] -a <BED/BAM/GFF/VCF> -b <BED/GFF/VCF>
| Option | Description |
|---|---|
| -a | BED/GFF/VCF file A. Each feature in A is compared to B in search of overlaps. Use “stdin” if passing A with a UNIX pipe. |
| -b | BED/GFF/VCF file B. Use “stdin” if passing B with a UNIX pipe. |
| -abam | BAM file A. Each BAM alignment in A is compared to B in search of overlaps. Use “stdin” if passing A with a UNIX pipe: For example: samtools view -b <BAM> | bedtools intersect -abam stdin -b genes.bed |
| -ubam | Write uncompressed BAM output. The default is write compressed BAM output. |
| -bed | When using BAM input (-abam), write output as BED. The default is to write output in BAM when using -abam. For example: bedtools intersect -abam reads.bam -b genes.bed -bed |
| -wa | Write the original entry in A for each overlap. |
| -wb | Write the original entry in B for each overlap. Useful for knowing what A overlaps. Restricted by -f and -r. |
| -loj | Perform a “left outer join”. That is, for each feature in A report each overlap with B. If no overlaps are found, report a NULL feature for B. |
| -wo | Write the original A and B entries plus the number of base pairs of overlap between the two features. Only A features with overlap are reported. Restricted by -f and -r. |
| -wao | Write the original A and B entries plus the number of base pairs of overlap between the two features. However, A features w/o overlap are also reported with a NULL B feature and overlap = 0. Restricted by -f and -r. |
| -u | Write original A entry once if any overlaps found in B. In other words, just report the fact at least one overlap was found in B. Restricted by -f and -r. |
| -c | For each entry in A, report the number of hits in B while restricting to -f. Reports 0 for A entries that have no overlap with B. Restricted by -f and -r. |
| -v | Only report those entries in A that have no overlap in B. Restricted by -f and -r. |
| -f | Minimum overlap required as a fraction of A. Default is 1E-9 (i.e. 1bp). |
| -r | Require that the fraction of overlap be reciprocal for A and B. In other words, if -f is 0.90 and -r is used, this requires that B overlap at least 90% of A and that A also overlaps at least 90% of B. |
| -s | Force “strandedness”. That is, only report hits in B that overlap A on the same strand. By default, overlaps are reported without respect to strand. |
| -S | Require different strandedness. That is, only report hits in B that overlap A on the _opposite_ strand. By default, overlaps are reported without respect to strand. |
| -split | Treat “split” BAM (i.e., having an “N” CIGAR operation) or BED12 entries as distinct BED intervals. |
| -sorted |
When using -sorted, memory usage remains low even for very large files. |
| -g | Specify a genome file the defines the expected chromosome order in the input files for use with the -sorted option. |
| -header | Print the header from the A file prior to results. |
5.1.17.2. Default behavior¶
By default, if an overlap is found, bedtools intersect reports the shared interval between the two overlapping features.
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed
chr1 15 20
5.1.17.3. -wa Reporting the original A feature¶
Instead, one can force bedtools intersect to report the original “A” feature when an overlap is found. As shown below, the entire “A” feature is reported, not just the portion that overlaps with the “B” feature.
For example:
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed -wa
chr1 10 20
5.1.17.4. -wb Reporting the original B feature¶
Similarly, one can force bedtools intersect to report the original “B” feature when an overlap is found. If just -wb is used, the overlapping portion of A will be reported followed by the original “B”. If both -wa and -wb are used, the originals of both “A” and “B” will be reported.
For example (-wb alone):
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed -wb
chr1 15 20 chr 15 20
Now -wa and -wb:
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed -wa -wb
chr1 10 20 chr 15 20
5.1.17.5. -loj Left outer join. Report features in A with and without overlaps¶
By default, bedtools intersect will only report features in A that have an overlap in B. The -loj option will report every A feature no matter what. When there is an overlap (or more than 1), it will report A with its overlaps. Yet when there are no overlaps, an A feature will be reported with a NULL B feature to indicate that there were no overlaps
For example (without -loj):
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed
chr1 10 20 chr 15 20
Now with -loj:
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed -loj
chr1 10 20 chr 15 20
chr1 30 40 . -1 -1
5.1.17.6. -wo Write the amount of overlap between intersecting features¶
The -wo option reports a column after each combination of intersecting “A” and “B” features indicating the amount of overlap in bases pairs that is observed between the two features.
Note
When an interval in A does not intersect an interval in B, it will not be reported. If you would like to report such intervals with an overlap equal to 0, see the -wao option.
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
chr1 18 25
$ bedtools intersect -a A.bed -b B.bed -wo
chr1 10 20 chr1 15 20 5
chr1 10 20 chr1 18 25 2
5.1.17.7. -wao Write amounts of overlap for all features.¶
The -wao option extends upon the -wo option in that, unlike -wo, it reports an overlap of 0 for features in A that do not have an intersection in B.
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
chr1 18 25
$ bedtools intersect -a A.bed -b B.bed -wao
chr1 10 20 chr1 15 20 5
chr1 10 20 chr1 18 25 2
chr1 30 40 . -1 -1 0
5.1.17.8. -u (unique) Reporting the mere presence of any overlapping features¶
Often you’d like to simply know a feature in “A” overlaps one or more features in B without reporting each and every intersection. The -u option will do exactly this: if an one or more overlaps exists, the A feature is reported. Otherwise, nothing is reported.
For example, without -u:
$ cat A.bed
chr1 10 20
$ cat B.bed
chr1 15 20
chr1 17 22
$ bedtools intersect -a A.bed -b B.bed
chr1 10 20
chr1 10 20
Now with -u:
$ cat A.bed
chr1 10 20
$ cat B.bed
chr1 15 20
chr1 17 22
$ bedtools intersect -a A.bed -b B.bed -u
chr1 10 20
5.1.17.9. -c Reporting the number of overlapping features¶
The -c option reports a column after each “A” feature indicating the number (0 or more) of overlapping features found in “B”. Therefore, each feature in A is reported once.
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
chr1 18 25
$ bedtools intersect -a A.bed -b B.bed -c
chr1 10 20 2
chr1 30 40 0
5.1.17.10. -v Reporting the absence of any overlapping features¶
There will likely be cases where you’d like to know which “A” features do not overlap with any of the “B” features. Perhaps you’d like to know which SNPs don’t overlap with any gene annotations. The -v (an homage to “grep -v”) option will only report those “A” features that have no overlaps in “B”.
$ cat A.bed
chr1 10 20
chr1 30 40
$ cat B.bed
chr1 15 20
$ bedtools intersect -a A.bed -b B.bed -v
chr1 30 40
5.1.17.11. -f Requiring a minimal overlap fraction¶
By default, bedtools intersect will report an overlap between A and B so long as there is at least one base pair is overlapping. Yet sometimes you may want to restrict reported overlaps between A and B to cases where the feature in B overlaps at least X% (e.g. 50%) of the A feature. The -f option does exactly this.
For example (note that the second B entry is not reported):
$ cat A.bed
chr1 100 200
$ cat B.bed
chr1 130 201
chr1 180 220
$ bedtools intersect -a A.bed -b B.bed -f 0.50 -wa -wb
chr1 100 200 chr1 130 201
5.1.17.12. -r, and -f Requiring reciprocal minimal overlap fraction¶
Similarly, you may want to require that a minimal fraction of both the A and the B features is overlapped. For example, if feature A is 1kb and feature B is 1Mb, you might not want to report the overlap as feature A can overlap at most 1% of feature B. If one set -f to say, 0.02, and one also enable the -r (reciprocal overlap fraction required), this overlap would not be reported.
For example (note that the second B entry is not reported):
$ cat A.bed
chr1 100 200
$ cat B.bed
chr1 130 201
chr1 130 200000
$ bedtools intersect -a A.bed -b B.bed -f 0.50 -r -wa -wb
chr1 100 200 chr1 130 201
5.1.17.13. -s Enforcing same strandedness¶
By default, bedtools intersect will report overlaps between features even if the features are on opposite strands. However, if strand information is present in both BED files and the “-s” option is used, overlaps will only be reported when features are on the same strand.
For example (note that the first B entry is not reported):
$ cat A.bed
chr1 100 200 a1 100 +
$ cat B.bed
chr1 130 201 b1 100 -
chr1 132 203 b2 100 +
$ bedtools intersect -a A.bed -b B.bed -wa -wb -s
chr1 100 200 a1 100 + chr1 132 203 b2 100 +
5.1.17.14. -S Enforcing opposite “strandedness”¶
The -s option enforces that overlaps be on the same strand. In some cases, you may want to enforce that overlaps be found on opposite strands. In this, case use the -S option.
For example:
$ cat A.bed
chr1 100 200 a1 100 +
$ cat B.bed
chr1 130 201 b1 100 -
chr1 132 203 b2 100 +
$ bedtools intersect -a A.bed -b B.bed -wa -wb -S
chr1 100 200 a1 100 + chr1 130 201 b1 100 -
5.1.17.15. -abam Default behavior when using BAM input (deprecated since 2.18.0)¶
When comparing alignments in BAM format (-abam) to features in BED format (-b), bedtools intersect will, by default, write the output in BAM format. That is, each alignment in the BAM file that meets the user’s criteria will be written (to standard output) in BAM format. This serves as a mechanism to create subsets of BAM alignments are of biological interest, etc. Note that only the mate in the BAM alignment is compared to the BED file. Thus, if only one end of a paired-end sequence overlaps with a feature in B, then that end will be written to the BAM output. By contrast, the other mate for the pair will not be written. One should use pairToBed(Section 5.2) if one wants each BAM alignment for a pair to be written to BAM output.
$ bedtools intersect -abam reads.unsorted.bam -b simreps.bed | \
samtools view - | \
head -3
BERTHA_0001:3:1:15:1362#0 99 chr4 9236904 0 50M = 9242033 5 1 7 9
AGACGTTAACTTTACACACCTCTGCCAAGGTCCTCATCCTTGTATTGAAG W c T U ] b \ g c e g X g f c b f c c b d d g g V Y P W W _
\c`dcdabdfW^a^gggfgd XT:A:R NM:i:0 SM:i:0 AM:i:0 X0:i:19 X1:i:2 XM:i:0 XO:i:0 XG:i:0 MD:Z:50
BERTHA _0001:3:1:16:994#0 83 chr6 114221672 37 25S6M1I11M7S =
114216196 -5493 G A A A G G C C A G A G T A T A G A A T A A A C A C A A C A A T G T C C A A G G T A C A C T G T T A
gffeaaddddggggggedgcgeggdegggggffcgggggggegdfggfgf XT:A:M NM:i:3 SM:i:37 AM:i:37 XM:i:2 X O : i :
1 XG:i:1 MD:Z:6A6T3
BERTHA _0001:3:1:16:594#0 147 chr8 43835330 0 50M =
43830893 -4487 CTTTGGGAGGGCTTTGTAGCCTATCTGGAAAAAGGAAATATCTTCCCATG U
\e^bgeTdg_Kgcg`ggeggg_gggggggggddgdggVg\gWdfgfgff XT:A:R NM:i:2 SM:i:0 AM:i:0 X0:i:10 X1:i:7 X M : i :
2 XO:i:0 XG:i:0 MD:Z:1A2T45
Note
As of version 2.18.0, it is no longer necessary to specify a BAM input file via -abam. Bedtools now autodetects this when -a is used.
5.1.17.16. -ubam Default behavior when using BAM input¶
The -ubam option writes uncompressed BAM output to stdout. This is useful for increasing the speed of pipelines that accept the output of bedtools intersect as input, since the receiving tool does not need to uncompress the data.
5.1.17.17. -bed Output BED format when using BAM input¶
When comparing alignments in BAM format (-abam) to features in BED format (-b), bedtools intersect will optionally write the output in BED format. That is, each alignment in the BAM file is converted to a 6 column BED feature and if overlaps are found (or not) based on the user’s criteria, the BAM alignment will be reported in BED format. The BED “name” field is comprised of the RNAME field in the BAM alignment. If mate information is available, the mate (e.g., “/1” or “/2”) field will be appended to the name. The “score” field is the mapping quality score from the BAM alignment.
$ bedtools intersect -abam reads.unsorted.bam -b simreps.bed -bed | head -20
chr4 9236903 9236953 BERTHA_0001:3:1:15:1362#0/1 0 +
chr6 114221671 114221721 BERTHA_0001:3:1:16:994#0/1 37 -
chr8 43835329 43835379 BERTHA_0001:3:1:16:594#0/2 0 -
chr4 49110668 49110718 BERTHA_0001:3:1:31:487#0/1 23 +
chr19 27732052 27732102 BERTHA_0001:3:1:32:890#0/2 46 +
chr19 27732012 27732062 BERTHA_0001:3:1:45:1135#0/1 37 +
chr10 117494252 117494302 BERTHA_0001:3:1:68:627#0/1 37 -
chr19 27731966 27732016 BERTHA_0001:3:1:83:931#0/2 9 +
chr8 48660075 48660125 BERTHA_0001:3:1:86:608#0/2 37 -
chr9 34986400 34986450 BERTHA_0001:3:1:113:183#0/2 37 -
chr10 42372771 42372821 BERTHA_0001:3:1:128:1932#0/1 3 -
chr19 27731954 27732004 BERTHA_0001:3:1:130:1402#0/2 0 +
chr10 42357337 42357387 BERTHA_0001:3:1:137:868#0/2 9 +
chr1 159720631 159720681 BERTHA_0001:3:1:147:380#0/2 37 -
chrX 58230155 58230205 BERTHA_0001:3:1:151:656#0/2 37 -
chr5 142612746 142612796 BERTHA_0001:3:1:152:1893#0/1 37 -
chr9 71795659 71795709 BERTHA_0001:3:1:177:387#0/1 37 +
chr1 106240854 106240904 BERTHA_0001:3:1:194:928#0/1 37 -
chr4 74128456 74128506 BERTHA_0001:3:1:221:724#0/1 37 -
chr8 42606164 42606214 BERTHA_0001:3:1:244:962#0/1 37 +
5.1.17.18. -split Reporting overlaps with spliced alignments or blocked BED features¶
As described in section 1.3.19, bedtools intersect will, by default, screen for overlaps against the entire span of a spliced/split BAM alignment or blocked BED12 feature. When dealing with RNA-seq reads, for example, one typically wants to only screen for overlaps for the portions of the reads that come from exons (and ignore the interstitial intron sequence). The -split command allows for such overlaps to be performed.
For example, the diagram below illustrates the default behavior. The blue dots represent the “split/ spliced” portion of the alignment (i.e., CIGAR “N” operation). In this case, the two exon annotations are reported as overlapping with the “split” BAM alignment, but in addition, a third feature that overlaps the “split” portion of the alignment is also reported.
- ::
Chromosome ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Exons ————— ———-
BED/BAM A ********.......................................****
BED File B ^^^^^^^^^^^^^^^ ^^^^^^^^ ^^^^^^^^^^
Result =============== ======== ==========
In contrast, when using the -split option, only the exon overlaps are reported.
- ::
Chromosome ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Exons ————— ———-
BED/BAM A ********.......................................****
BED File B ^^^^^^^^^^^^^^^ ^^^^^^^^ ^^^^^^^^^^
Result =============== ==========
5.1.17.19. -sorted Invoke a memory-efficient algorithm for very large files.¶
The default algorithm for detecting overlaps loads the B file into an R-tree structure in memory. While fast, it can consume substantial memory for large files. For these reason, we provide an alternative, memory efficient algorithm that depends upon inout files that have been sorted by chromosome and then by start position. When both input files are position-sorted, the algorithm can “sweep” through the data and detect overlaps on the fly in a manner much like the way database systems join two tables. This option is invoked with the -sorted option.
Note
By default, the -sorted option requires that the records are GROUPED by chromosome and that within each chromosome group, the records are sorted by chromosome position. One way to achieve this (for BED files for example) is use the UNIX sort utility to sort both files by chromosome and then by position. That is, sort -k1,1 -k2,2n in.bed > in.sorted.bed. However, since we merely require that the chromsomes are grouped (that is, all records for a given chromosome come in a single block in the file), sorting criteria other than the alphanumeric criteria that is used by the sort utility are fine. For example, you could use the “version sort” (-V) option in newer versions of GNU sort to make the chromosomes come in this (chr1, chr2, chr3) order instead of this (chr1, chr10, chr11) order.
For example:
$ bedtools intersect -a big.sorted.bed -b huge.sorted.bed -sorted
5.1.17.20. -g Define an alternate chromosome sort order via a genome file.¶
As described above, the -sorted option expects that the input files are grouped by chromosome. However, there arise cases where ones input files are sorted by a different criteria and it is to computationally onerous to resort the files alphanumerically. For example, the GATK expects that BAM files are sorted in a very specific manner. The -g option allows one to specify an exact ording that should be expected in the input (e.g., BAM, BED, etc.) files. All you need to do is re-order you genome file to specify the order. Also, the use of a genome file to specify the expected order allows the intersect tool to detect when two files are internally grouped but each file actually follows a different order. This will cause incorrect results and the -g file will alert you to such problems.
For example, an alphanumerically ordered genome file would look like the following:
$ cat hg19.genome
chr1 249250621
chr10 135534747
chr11 135006516
chr12 133851895
chr13 115169878
chr14 107349540
chr15 102531392
chr16 90354753
chr17 81195210
chr18 78077248
chr19 59128983
chr2 243199373
chr20 63025520
chr21 48129895
chr22 51304566
chr3 198022430
chr4 191154276
chr5 180915260
chr6 171115067
chr7 159138663
chr8 146364022
chr9 141213431
chrM 16571
chrX 155270560
chrY 59373566
However, if your input BAM or BED files are ordered such as chr1, chr2, chr3, etc., one need to simply reorder the genome file accordingly:
$ sort -k1,1V hg19.genome > hg19.versionsorted.genome
$ cat hg19.versionsorted.genome
chr1 249250621
chr2 243199373
chr3 198022430
chr4 191154276
chr5 180915260
chr6 171115067
chr7 159138663
chr8 146364022
chr9 141213431
chr10 135534747
chr11 135006516
chr12 133851895
chr13 115169878
chr14 107349540
chr15 102531392
chr16 90354753
chr17 81195210
chr18 78077248
chr19 59128983
chr20 63025520
chr21 48129895
chr22 51304566
chrM 16571
chrX 155270560
chrY 59373566
At this point, one can now use the -sorted option along with the genome file in order to properly process the input files that abide by something other than an alphanumeric sorting order.
$ bedtools intersect -a a.versionsorted.bam -b b.versionsorted.bed \
-sorted \
-g hg19.versionsorted.genome
Et voila.
5.1.17.21. -header Print the header for the A file before reporting results.¶
By default, if your A file has a header, it is ignored when reporting results. This option will instead tell bedtools to first print the header for the A file prior to reporting results.
